马дёҠжіЁеҶҢпјҢз»“дәӨжӣҙеӨҡеҘҪеҸӢпјҢдә«з”ЁжӣҙеӨҡеҠҹиғҪпјҢи®©дҪ иҪ»жқҫзҺ©иҪ¬зӨҫеҢәгҖӮ
жӮЁйңҖиҰҒ зҷ»еҪ• жүҚеҸҜд»ҘдёӢиҪҪжҲ–жҹҘзңӢпјҢжІЎжңүиҙҰеҸ·пјҹз«ӢеҚіжіЁеҶҢ

x
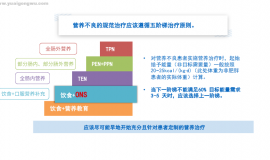
VCжҠ—зҷҢиҜҙз”ұжқҘе·Ід№…пјҢдј—иҜҙзә·зәӯпјӣдҪҶеӨ§еӨҡжҳҜз»ҶиғһгҖҒеҠЁзү©иҜ•йӘҢжҲ–дёӘдҫӢжҠҘе‘ҠпјҢдёҚи¶ідёәеҮӯгҖӮ: d7 a9 ~4 `6 o9 ]
2 K) U# g/ _6 Z; z0 VзҺ°жҗңйӣҶж•ҙзҗҶдёӨдёӘVCжҠ—зҷҢзҡ„дёҙеәҠиҜ•йӘҢеҰӮдёӢпјҢжҚ®жӯӨеҠ д»Ҙз ”еҲӨпјҡ3 V. m& I7 e- e, x! b
) ~1 W0 w9 I: q" R3 E5 T) B8 f
+ x e- A( g1 M$ Q# |- C6 w. X- M: a: G5 c
1гҖҒгҖҠA Randomized, Open-Label, Multicenter, Phase 3 Study of High-Dose Vitamin C Plus FOLFOX Вұ Bevacizumab versus FOLFOX Вұ Bevacizumab in Unresectable Untreated Metastatic Colorectal Cancer (VITALITY Study)гҖӢ
! ?6 q' i1 G# e7 d5 E3 \- A ' s6 p) `9 q8 W& N0 h+ |
иҝҷеә”иҜҘжҳҜеҲ°зӣ®еүҚдёәжӯўпјҢж¶үеҸҠеҲ°VCжҠ—зҷҢзҡ„пјҢ规模жңҖеӨ§зҡ„дёҖдёӘйҡҸжңәеҜ№з…§иҜ•йӘҢгҖӮ
) P' `0 s/ P6 G$ Z# S 9 _+ p% M2 d0 w$ Q# u) I! h! @0 R
Patients and methods: Between 2017 and 2019, histologically confirmed patients with mCRC (n = 442) with normal glucose-6-phosphate dehydrogenase status and no prior treatment for metastatic disease were randomized (1:1) into a control (FOLFOX Вұ bevacizumab) and an experimental [high-dose vitamin C (1.5 g/kg/d, intravenously for 3 hours from D1 to D3) plus FOLFOX Вұ bevacizumab] group. Randomization was based on the primary tumor location and bevacizumab prescription.) ^' j# u2 f# `. L" `3 D
+ Y% a7 z [2 d3 v; a5 P" s: ?иҜ•йӘҢз»“жһңпјҡThe progression-free survival (PFS) of the experimental group was not superior to the control group [median PFS, 8.6 vs. 8.3 months; HR, 0.86; 95% confidence interval (CI), 0.70-1.05; P = 0.1]. The objective response rate (ORR) and overall survival (OS) of the experimental and control groups were similar (ORR, 44.3% vs. 42.1%; P = 0.9; median OS, 20.7 vs. 19.7 months; P = 0.7). Grade 3 or higher treatment-related adverse events occurred in 33.5% and 30.3% of patients in the experimental and control groups, respectively.
1 Q% P% s1 m8 Q+ N O6 r+ |0 Y) i) d; `
$ [, {, m% [5 x3 TиҝҷдёӘдёҙеәҠиҜ•йӘҢз»“жһңж„Ҹе‘ізқҖеҚідҫҝжҳҜйҮҮз”ЁдәҶйқҷи„үжіЁе°„зҡ„з»ҷиҚҜж–№ејҸпјҢеҚідҫҝжҳҜз”ЁдәҶ1.5 g/kg/dиҝҷд№Ҳй«ҳзҡ„еүӮйҮҸпјҲдёҖдёӘ50е…¬ж–Өзҡ„жӮЈиҖ…дёҖеӨ©иҰҒйқҷи„үжіЁе°„75е…Ӣд№ӢеӨҡзҡ„VCпјүпјҢд»Һж•ҙдҪ“иҖҢиЁҖпјҢдј з»ҹеҢ–з–—йқ¶еҗ‘жҠ—зҷҢжІ»з–—еўһеҠ IVCпјҢд№ҹжІЎжңүз»ҷз–—ж•ҲеёҰжқҘжңүз»ҹи®ЎеӯҰж„Ҹд№үдёҠзҡ„ж”№е–„гҖӮ
5 W4 x8 I6 b% O* c8 Y
- Q+ p. K" _" PдҪҶжҳҜпјҢвҖңIn prespecified subgroup analyses, patients with RAS mutation had significantly longer PFS (median PFS, 9.2 vs. 7.8 months; HR, 0.67; 95% CI, 0.50-0.91; P = 0.01) with vitamin C added to chemotherapy than with chemotherapy only.вҖқ1 o% k' v* {, w" Y; L. M
$ |8 g4 W) k3 Z: {0 VеңЁRASзӘҒеҸҳпјҲkrasгҖҒhrasгҖҒnrasпјүжӮЈиҖ…дёӯпјҢеә”з”Ёйқҷи„үжіЁе°„еӨ§еүӮйҮҸVCпјҢеҚҙеёҰжқҘдәҶPFSзҡ„з»ҹи®ЎеӯҰж„Ҹд№үдёҠзҡ„жҳҫи‘—ж”№е–„гҖӮ+ q, f% E7 _5 U% `
% [ X2 r7 e: e4 M" u) H. Xз ”з©¶иҖ…и®ӨдёәиҝҷдёӘз»“жһңд№ҹжҳҜи·ҹдёҙеәҠеүҚз ”з©¶з»“и®әзӣёз¬Ұзҡ„ пјҡвҖңIt suggested that the oxidized form of vitamin C, dehydroascorbate, was the pharmaceutically active agent resulting in an energy crisis and colorectal cancer cell death, and the selective cytotoxicity of vitamin C stemmed from high expression of GLUT1 glucose transporter combined with RAS oncogene-induced glycolytic addictionвҖқ
) L; ?, `' H- g& l; z# o m0 j& I
6 w) q2 ]' }5 Q# E0 CеҸҰеӨ–еңЁи¶…иҝҮ55еІҒзҡ„иҖҒе№ҙжӮЈиҖ…дёӯд№ҹеёҰжқҘдәҶPFSдёҠзҡ„зӣҠеӨ„гҖӮиҝҷжҲ–и®ёи·ҹиҖҒе№ҙжӮЈиҖ…дёӯVCзјәд№ҸжҜ”дҫӢй«ҳпјҲ88%пјүжңүдёҖе®ҡзҡ„е…ізі»гҖӮ
1 r! K1 ?) s e% W& F5 a ; Y% b/ y/ s! g0 H# w
з ”з©¶иҖ…и®ӨдёәдёҙеәҠи®ҫи®ЎдёҠжңүдёӨдёӘдёҚи¶іпјҡвҖңFirst, the patients received intravenous high-dose vitamin C for 3 days of every treatment cycle, which might not be enough for vitamin C to show its antitumor effect.вҖқвҖңSecond, high-dose vitamin C discontinued at 6 months before the majority of patients progressed, and the true impact of high-dose vitamin C in mCRC may thus be underestimated.вҖқ+ p3 C( X6 a$ r% D! R$ k$ h
' D% G$ j( G0 O! T
ж ёеҝғж„ҸжҖқе°ұжҳҜе°Ҫз®ЎIVCзҡ„еҚ•ж—ҘеүӮйҮҸдёҚе°ҸпјҢдҪҶжҳҜжү“зҡ„еӨ©ж•°дёҚеӨҡпјҢжІ»з–—з§ҜзҙҜзҡ„жҖ»йҮҸ并дёҚз®—еҫҲеӨҡпјҢжңүеҸҜиғҪжІЎжңүи®©VCеҸ‘жҢҘеҮәеә”жңүзҡ„дҪңз”ЁжқҘгҖӮ) v5 A" ~% g' j! l( R1 c) x
% B+ U. ~$ Y# P' F$ n6 I* Lд»ҺиҝҷдёӘдёҙеәҠиҜ•йӘҢз»“жһңжқҘзңӢпјҢVCиҰҒеҸ‘жҢҘжҠ—зҷҢдҪңз”ЁпјҢе…ій”®еңЁдәҺжӮЈиҖ…зҡ„еҹәеӣ зӘҒеҸҳжғ…еҶөпјҢеңЁдәҺжӮЈиҖ…зҡ„VCзјәд№Ҹжғ…еҶөпјҢеңЁдәҺVCзҡ„з»ҷиҚҜж–№ејҸгҖҒеүӮйҮҸгҖҒеүӮеһӢгҖҒз»ҷиҚҜйў‘зҺҮзҡ„жғ…еҶөгҖӮдёҚиғҪи„ұзҰ»иҝҷдәӣе…·дҪ“жқЎд»¶еҺ»еҫ—еҮәд»Җд№Ҳз»“и®әгҖӮ
8 d' R# x. V0 h W5 n( l5 h 3 l6 c, z/ m1 v& ?
дёӢйқўиҝҷдёӘдёҙеәҠиҜ•йӘҢд№ҹиҜҙжҳҺдәҶиҝҷзӮ№гҖӮ
, k5 r( }* x8 ?( K; x5 G8 ?2 ~
4 j$ \7 `1 e2 w h8 D+ N2гҖҒгҖҠRandomized trial of topical ascorbic acid in DMSO versus imiquimod for the treatment of basal cell carcinomaгҖӢ' H+ L0 e" L6 l5 n! T8 F
& @' s) N& Q" {6 p
иҝҷжҳҜеҲ°зӣ®еүҚдёәжӯўпјҢVCжҠ—зҷҢз–—ж•ҲжңҖеҘҪзҡ„дёҖдёӘдёҙеәҠиҜ•йӘҢгҖӮ
$ w/ \$ ]+ F0 b3 D' V3 @2 \9 G
) T0 a; F+ e- I/ kвҖңThe objective of this study was to compare efficacy of a topical solution consisting of 30% ascorbic acid in 95% dimethylsulfoxide with topical imiquimod in the treatment of basal cell carcinoma. Twenty-five patients with 29 biopsy confirmed basal cell carcinomas were randomly assigned to receive either the topically applied ascorbic acid treatment twice daily for 8 weeks or topical imiquimod, a standard and well characterized topical treatment. вҖқ
) |5 _) H! }$ y6 q( {* \ , R$ ~% q; A+ R* l
иҜ•йӘҢз»“жһңпјҡвҖңAfter 8 weeks, post-treatment biopsy of lesions showed complete resolution of 13/15 (86.7%) in the ascorbic acid group, while 8/14 (57.1%) lesions in the IMQ group were resolved (p < 0.05 Chi Square). Topical ascorbic acid was superior at 8 weeks, and non-inferior at 12 weeks to topical imiquimod in the treatment of low risk nodular and superficial lesions. In addition, ascorbic acid was associated with fewer adverse effects than imiquimod. 70% of patients in the imiquinod group showed residual hypopigmentation at 30mo follow up versus 0% in the ascorbate group.вҖқ
+ m/ {2 A! Q+ f9 M
& b2 `$ a' U0 b' k* K/ e$ `/ cеүӮеһӢдёҠжҠҠascorbic acid жә¶еңЁ DMSOиҝҷдёӘжё—йҖҸеҠӣжһҒејәзҡ„ж— жҜ’зҡ„дҪҗеүӮйҮҢпјҢз»ҷиҚҜж–№ејҸдёҠйҮҮз”Ёж¶ӮжҠ№иҝҷз§ҚиҚҜзү©зӣҙжҺҘжҺҘи§Ұз—…зҒ¶зҡ„ж–№ејҸпјҢиҝҷж ·дҝқиҜҒдәҶиҚҜзү©еңЁз—…зҒ¶еҶ…зҡ„еұҖйғЁй«ҳжө“еәҰпјҢиҝҷжҳҜдёҙеәҠиҜ•йӘҢжҲҗеҠҹзҡ„е…ій”®гҖӮ4 k/ E$ I- [. A1 \# |
2 j7 S$ x% w. b7 dжҢүз…§иҝҷдёӨдёӘдёҙеәҠиҜ•йӘҢзҡ„жҖқи·ҜпјҢе®ҢжҲҗеҸҜд»ҘжӢ“еұ•VC-DMSOиҚҜж¶ІеҜ№RASзӘҒеҸҳжӮЈиҖ…жҲ–иҖ…дёҘйҮҚзјәд№ҸVCжӮЈиҖ…з—…зҒ¶е®һж–Ҫз—…зҒ¶иЎЁйқўз»ҷиҚҜжҲ–иҖ…зҳӨдҪ“еҶ…зӣҙжҺҘз»ҷиҚҜзҡ„жІ»з–—гҖӮ8 F4 w5 s! u1 \2 w# r* a: n5 K# J) @
|